How we split the atom for a television programme
By Tim Usborne, Producer/Director
To help television audiences understand the story we were attempting to tell in , we needed to recreate Otto Hahn’s famous atom-splitting nuclear fission experiment from 1938.
As we researched the complexities of performing the experiment, we were struck by a sobering thought: as far as we could tell, no one had ever performed this experiment for TV before.
And for good reason! Unlike the 1930s, nowadays many of the elements needed to make the experiment work are controlled by the government.
Uranium; radiation detectors; neutrons beams — not the kind of paraphernalia that sits around in many laboratories, because the explosive energy they can theoretically unleash is enormous.
These are not the kinds of materials anyone would want to fall into the wrong hands.
We located one laboratory that had uranium, but no beam. One had both, but not enough uranium — it turned out that the fission reaction was actually so rare that at least a gram of uranium was needed to begin to see anything.
Then we found a beam and the uranium, but the beam was too powerful: ironically, it produced neutrons that were too energetic to split the atoms and, anyway, the lab wasn’t properly licensed to perform our experiment.
It seemed as though Otto Hahn might elude us.
Then, a week before we were hoping to shoot, we received a call from .
Deep in the bowels of his lab was a massive proton accelerator – a huge device used for a range of scientific experiments. Paddy reckoned that, if we were lucky, we could get access to this very busy beam and use it to create the neutrons we needed. What’s more, he also thought he had a lump of uranium we could bombard with them. If the lump was large enough, after an hour or so, enough atoms would have split – ‘fissioned’ – to see some results.
Could this finally be the break we were after?
Our experiment
On a bright March morning at NPL in Teddington, the TV production team and presenter began filming Paddy and his team as they set up the experiment.

A thin sliver – just over a gram – of uranium was lowered into the beam. The huge accelerator was switched on. The proton accelerator began to produce neutrons.
Now all we had to do was wait.
If Paddy’s calculations were right – and as the experiment hadn’t been performed for over 60 years he wasn’t entirely confident! – a few of the neutrons bombarding the uranium nucleus should cause it to fission and the telltale outcome would be a small but measurable amount of the element barium.
After an hour, Jim switched off the beam and carefully removed the uranium.
Then he checked the amount of radioactivity of the sample: the Geiger counter registered off the meter. This was a good sign as it meant the neutrons had definitely begun to hit their targets – the nuclei themselves – causing them to become slightly unstable and spit out tiny nuclear fragments.
But had the atoms actually split?
Carefully, the sliver was placed inside a spectrometer — a device that can measure the signature of the atoms inside it. The machine whirred and clicked. If it worked it would, as far as we knew, be the first time ever on British television that an atom had been split.
Suddenly, the computers beeped: the results were in.
There, nestling on a simple graph, was the result that Otto Hahn had seen over 70 years before. It wasn’t much to look at – merely a small yellow bump in a graph on a computer screen – but that bump indicated the presence of barium, which meant that our uranium had split. It had undergone nuclear fission, unlocking some of the most powerful forces in the universe.
That tiny yellow bump meant that we were witnessing our own version of the Dawn the Atomic Age.
Otto Hahn and the birth of the nuclear age
Late in 1938, as the tide of war began to flow Europe, a chemist worked through the night in his laboratory in Berlin.

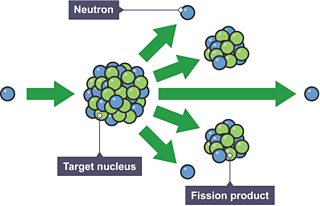
Otto Hahn was bombarding atoms with small particles called neutrons. These particles had the curious ability to penetrate deep into the core of an atom – into its nucleus of protons and neutrons – and subtly change its properties. He and his colleagues carefully catalogued the slight subtle differences of a range of bombarded elements; it was important work, if slightly routine.
As he bombarded a metal called uranium, he expected to create a slightly heavier nucleus, perhaps with 239 or 240 particles instead of its usual 238. Instead, the result defied rational explanation: the experiment produced barium, an element whose nucleus contains around half the number of particles — about 137.
How could adding one neutron to uranium end up producing an atom half the size?
Hahn was flummoxed and assumed he had made some experimental error. He wrote to his colleague and friend, , asking if she could make head or tail of his results.
Meitner was equally baffled at first, but she eventually came to the shocking realisation that the neutrons hitting the uranium were causing its nucleus to burst — something no one had imagined possible. It was a process that came to be known as .
Meitner calculated that the nucleus splitting released a fantastic burst of energy. One single atom bursting released enough energy to move a grain of sand, which might not sound like much until you consider that each grain of sand contains a billion, billion atoms!
. In splitting the atom, he unwittingly and single-handedly unlocked one of the most powerful forces in the universe and triggered the birth of the nuclear age.
-
![]()
Watch the programme: Monday 10 August at 9pm on 主播大秀 Four

